Timing - The key to the successful application of insecticides for Codling moth control
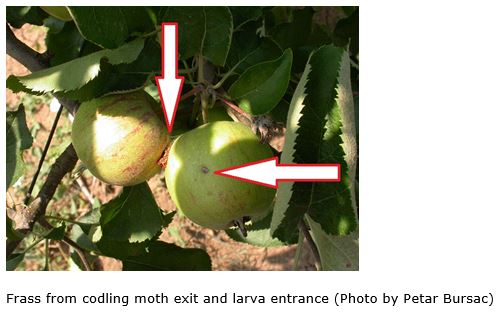
One of the most dangerous pests of apples that an orchardist or gardener is faced with is codling moth. Besides apples, also attacks pears, quinces, and occasionally walnuts and stone fruits. It is a hard pest to control because once the grub is inside the apple it is safe from insecticides and predators.
Management programs for Codling moth are based on preventing larval entry into the fruit. These programs are timed by heat-driven models that are synchronized to field populations by first capture of overwintering moths in pheromone traps. Proper timing of insecticide sprays is critical. Insecticides should be applied before or just as eggs are hatching. Once the worm has gone into the fruit or nut, it is protected from pesticides. If insecticides are applied too late, larvae will have tunneled into the fruit where they can no longer be controlled by insecticides.
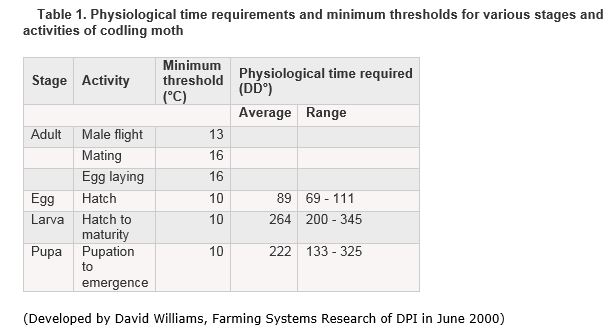
Pheromone traps are used to establish the biofix (biological fix point) for the model. The life stage and relative size of a codling moth population can be determined using the degree day model and pheromone traps. Pheromone traps should be checked twice per week to determine biofix, or the initial emergence of over-wintering first generation moths. Biofix occurs when there is an average of more than three moths caught in a trap over a 3- to 5-day period. For the rest of the season, these traps can be an effective way to monitor not only population cycles, but also the relative success of insecticide sprays. Trap counts can be used in combination with degree-days (DD) to accurately pinpoint the stage of moth development.
The degree day model is based on a developmental threshold of 10˚C for moth development and works as follows:
- Begin calculating degree-days at biofix.
- Calculate the maximum and minimum temperatures for each day and fit them into the following equation: [(Max + Min)/2]-10.
- The daily DD counts should be summed and accumulated as the season progresses.
This season we have experienced high temperatures which caused increased activity of Codling moth. Growers should pay more attention on this pest, check monitoring traps regularly, determine biofix dates, hatching and spraying dates and make appropriate decision on chemicals used for spraying.
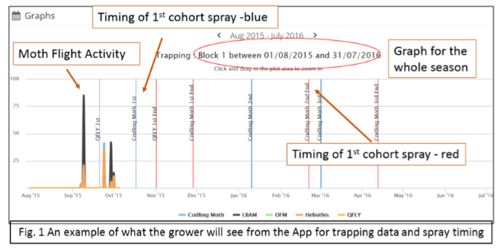
To make Codling moth management easier and more precise, Fruit Growers Victoria has developed a software, GrowFruit App which is a web based tool that uses the Phenological degree day (DD) modelling for the management of the key moths (Codling Moth; Oriental Fruit Moth and Light Brown Apple Moth) in Pome and Stonefruit production.
The application works on entering trapping data to establish a biofix date (i.e. first sign of new season activity). From this acquired information, data is fed into the program and using historical and forecast data from either a dedicated weather station on farm or a nearby Bureau of Meteorology station, a recommended spray date application is created and the period of cover the grower will need to apply it for that particular generation. This includes the CM 1st generation for both the 1st and 2nd Cohort emergence, which can catch many growers out.
(To find out more about the App and what it can deliver and to be able to access the program for this season please contact FGV on 0358253700; or
Codling moth can be very difficult to manage, especially if the population has been allowed to build up over a season or two. It is much easier to keep moth numbers low from the start than to suppress a well-established population.
An IPM program for codling moth management consists of different tools such as insecticides, mating disruption, and cultural controls. Mating disruption is the preferred tool because of its low toxicity to people, natural enemies, and the environment, but it may need to be supplemented with insecticide sprays, especially during the first few years.
Cultural methods for Codling Moth management include pruning and thinning, removal of alternate hosts and removal of dropped fruit. Pruning and thinning allows insecticide spray penetration and coverage. Pear and walnut trees should either be removed from sites near the orchard, or included in any codling moth treatments applied to the rest of the orchard. Apples that will not be picked from young nursery trees should be removed early in the season. Raking and removing apples on the ground will prevent larvae maturing in dropped apples. Cardboard wrapping around the trunk and main branches can be used as non-chemical method for reducing of population of Codling moth. This provides a perfectly shaped place for the grub to spin its cocoon. This band needs to be taken off and destroyed.
Combinations of soft chemicals and pheromone-based mating disruption have proven effective in control of Codling moth. Use of lower toxicity and selective insecticides enhances populations of beneficials.
Mating disruption is a technique, where the female sex pheromone of the codling moth is released in enormous quantities in the orchard, preventing the male moth from locating the female for mating. This technique is safe for non-target insects and doesn’t leave pesticide residues on the fruit. In orchards under a regular spray program, pheromone traps loaded with a 1-mg lure and placed in the lower to mid-level of the canopy are sufficient for monitoring purposes. However, in mating disruption blocks the pheromone traps should be loaded with a 5 to 10 mg lure, and the traps should be placed in the upper canopy of the tree.
Among insecticides generally used for codling moth control, there are none with enough selectivity and effectiveness to be useful for integrated control programs. During the 1980's, the agricultural chemical industry made a substantial effort to develop selective insecticides for codling moth control. Development efforts focused on insect growth regulators such as the chitin-synthesis inhibitors and juvenile hormone mimics. During the 1990’s, a new group of chemicals referred to as ecdysone-agonists, are highly specific, affecting only Lepidoptera. The advantage of IGRs is that they are more selective than conventional neuroactive insecticides and have few, if any, detrimental impacts on natural enemies of pests.
There is a number of new molecules with good selectivity known as pro-insecticides. Because of its pro-insecticide properties, Sodium Channel Blocking Insecticides require conversion into the active insecticidal form by the action in the body of the insect. These insecticides have proven to be relatively benign to natural enemies. Codling moth larvae treated with Sodium Channel Blocking Insecticides produce neurotoxic symptoms, beginning with ataxia and feeding cessation followed by tremors, mild convulsions and progressing to a flaccid paralysis. Molt accelerating compounds (MACs) are a novel class of insecticides that kill by disrupting the normal moulting processes of insects. Ingestion of even a minute amount of MACs causes grubs and caterpillars to attempt a premature, lethal moult. New group of insecticides, Ryanodine Receptor (RyR) Effectors or Calcium Homeostasis Insecticides, have low impact on most key beneficial insects and they are commonly used in Australia for Codling moth control.
Among the new options available for multi-tactic Codling moth control is a naturally occurring virus know as a Cydia pomonella granulovirus (CpGV). Rotating it with chemical insecticides is a good means of combating resistance. This virus is highly specific to Codling moth and it is non-infectious towards beneficial insects, wildlife and humans. When using CpGV, the following factors must be kept in mind:
1) CpGV must be ingested by Codling moth larva and may not kill it immediately;
2) the virus breaks down in the environment, thus a spray may not be effective for a week or so, and
3) the virus is highly lethal, a few protein occlusion bodies are all that is required to cause death.
In order not to lose the effectiveness of the insecticides, growers need to pay attention on resistance management.
Resistance to insecticides may be defined as a heritable change in the sensitivity of a pest population that is reflected in the repeated failure of a product. To achieve the expected level of control when used according to the label recommendation for that pest species.
Resistance arises through the overuse or misuse of an insecticide or acaricide against a pest species and results in the selection of resistant forms of the pest and the consequent evolution of populations that are resistant to that insecticide or acaricide.
In most cases, not only does resistance render the selecting compound ineffective but it often confers cross-resistance to other chemically related compounds. This is because compounds within a specific chemical group usually share a common target site within the pest and thus share a common mode of action (MoA). It is common for resistance to develop that is based on a genetic modification of this target site. When this happens, the interaction of the selecting compound with its target site is impaired and the compound loses its efficacy. Because all compounds within the chemical sub-group share a common MoA, there is a high risk that the resistance that has developed will automatically confer crossresistance to all the compounds in the same sub-group. It is this concept of cross-resistance within chemically related insecticides or acaricides that is the basis of the IRAC Mode of Action Classification.
The objective of successful Insecticide Resistance Management is to prevent or delay the evolution of resistance to insecticides, or to help regain susceptibility in insect pest populations in which resistance has already appeared. Effective IRM is thus an important element in maintaining the efficacy of valuable insecticides.
In practice, alternations, sequences, rotations or even mixtures of compounds from different MoA groups provide a sustainable and effective approach to IRM. This ensures that selection from compounds in any one MoA group is minimized. The IRAC classification in this chapter is provided as an aid to insecticide selection for these types of IRM strategies.
Recommendations for successful resistance management (Modern Crop Protection Compounds, Wolfgang Kramer and Ulrich Schirmer):
- Consult a local agricultural advisor or extension service in the area for up-to-date recommendations and advice on IPM and IRM programs
- Consider options for minimizing insecticide use by selecting early-maturing or pesttolerant varieties of crop plants
- Include effective cultural and biological control practices that work in harmony with effective IRM programs. Adopt all non-chemical techniques known to control or suppress pest populations, including biological sprays such as Bt’s, resistant varieties, within-field refuges (untreated areas) and crop rotation
- Where possible select insecticides and other pest management tools that preserve beneficial insects
- Use products at their full, recommended doses. Reduced (sub-lethal) doses quickly select populations with average levels of tolerance, whilst doses that are too high may impose excessive selection pressures
- Appropriate, well-maintained equipment should be used to apply insecticides. Recommended water volumes, spray pressures and optimal temperatures should be used to obtain optimal coverage
- Where larval stages are being controlled, target younger larval instars where possible because these are usually much more susceptible and therefore much more effectively controlled by insecticides than older ones
- Use appropriate local economic thresholds and spray intervals
- Follow label recommendations or local expert advice for use of alternations or sequences of different classes of insecticides with differing modes of action as part of an IRM strategy
- Where there are multiple applications per year or growing season, alternate products of different MoA classes
- In the event of a control failure, do not reapply the same insecticide but change the class of insecticides to one having a different mode of action and to which there is no [locally] known cross-resistance
- Mixtures may offer a short-term solution to resistance problems, but it is essential to ensure that each component of a mixture belongs to a different insecticide mode of action class, and that each component is used at its full rate
- Consideration should be given to monitor the incidence of resistance in the most commercially important situations and gauge levels of control obtained
- Withholding use of a product to which resistance has developed until susceptibility returns may be a valid tactic if sufficient alternative chemical classes remain to provide effective control.
Petar Bursac, Fruit Growers Victoria
References:
- David Williams, Codling Moth, Note Number: AG0095, Knoxfield, June 2000
- Flint, M. L. 1998. Pests of the Garden and Small Farm: A Grower’s Guide to Using Less Pesticide, 2nd ed. Oakland: Univ. Calif. Agric. Nat. Res. Publ. 3332.
- Hollingsworth, C.S. (Ed.), Codling Moth, Pacific Northwest Insect Management Handbook, Oregon State University, 2015
- J. L. Caprile, P. M. Vossen, Codling Moth, Revised 5/11, University of California Agriculture and Natural Resoruces, Statevide Integrated Pest Management Program, Davis, CA 95616
- Larry Gut, Codling moth control using granulosis virus, Fruit Crop Advisory Team Alert, Vol. 20, No. 8, Michigan State University, May 31, 2995
- Ohlendorf, B. 1999. Integrated Pest Management for Apples & Pears, 2nd ed. Oakland: Univ. Calif. Agric. Nat. Res. Publ. 3340.
- Wolfgang Kramer; Modern Crop Protection Compounds, John Wiley & Sons, 2007